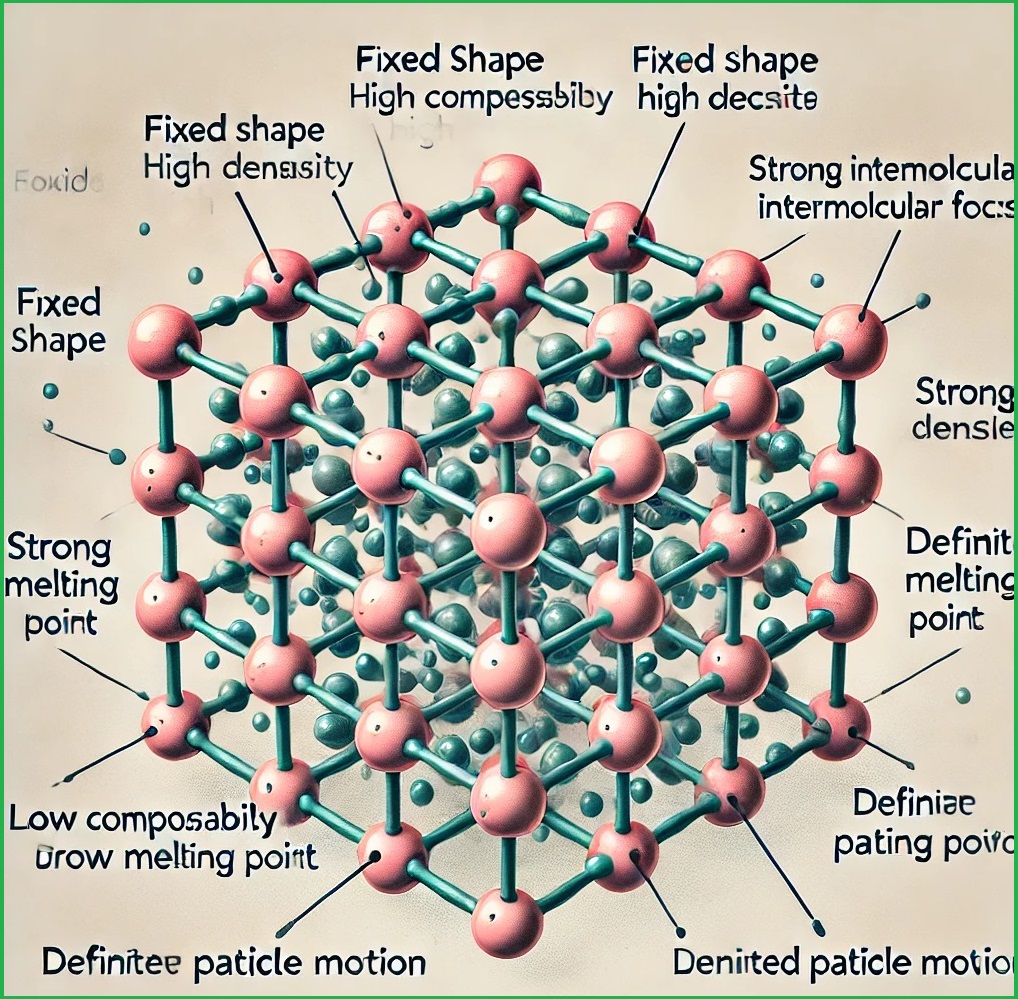
The solid state of matter has several unique characteristics that distinguish it from other states, like liquids and gases. Here are the general characteristics of the solid state:
1. Definite Shape and Volume
- Solids have a fixed, definite shape and volume due to the strong forces of attraction between particles. Unlike liquids and gases, which can flow and take the shape of their container, solids maintain a rigid structure unless acted upon by an external force.
2. Arrangement of Particles
- Crystalline Solids: These have a highly ordered and repeating arrangement of particles, forming a crystal lattice structure. Examples include table salt, metals, and gemstones like diamonds.
- Amorphous Solids: These solids lack a long-range order in their structure, making them less organized than crystalline solids. They do not have a sharp melting point and may soften over a range of temperatures. Examples include glass, rubber, and plastic.
3. High Density and Incompressibility
- The particles in a solid are packed very closely together, resulting in high density. Solids are also nearly incompressible, as the particles have little free space between them to be pushed closer together.
4. Low Rate of Diffusion
- Diffusion in solids is extremely slow compared to liquids and gases. This is because particles in a solid are tightly bound and only have limited movement, so they can’t easily spread out over time.
5. Thermal Expansion
- Solids expand when heated, though this expansion is usually small compared to liquids and gases. Heating increases particle vibrations within the solid structure, slightly increasing the distances between particles.
6. Mechanical Properties
- Elasticity: Solids can temporarily deform when force is applied but return to their original shape once the force is removed, up to a certain limit.
- Plasticity: Beyond a certain limit, solids can experience permanent deformation.
- Hardness: Hardness is a measure of a solid’s resistance to deformation or scratching.
- Brittleness and Ductility: Some solids, like glass, are brittle and break under stress, while others, like metals, are ductile and can be stretched or drawn into wires.
7. Electrical Conductivity
- Solids vary in their ability to conduct electricity:
- Conductors: Metals conduct electricity due to the presence of free electrons that can move through the structure.
- Insulators: Materials like rubber and glass do not conduct electricity because they lack free charge carriers.
- Semiconductors: Solids like silicon have intermediate conductivity, which can be enhanced by introducing impurities, known as doping, for use in electronics.
8. Thermal Conductivity
- Solids generally conduct heat due to the close packing of particles, which allows energy to transfer more easily through the material. Metals are especially good thermal conductors, while some solids, like wood, are poor conductors.
9. Magnetic Properties
- Solids can exhibit various magnetic behaviors:
- Diamagnetic: Weakly repelled by a magnetic field (e.g., copper, gold).
- Paramagnetic: Weakly attracted to a magnetic field (e.g., aluminum).
- Ferromagnetic: Strongly attracted and able to retain magnetic properties even when the magnetic field is removed (e.g., iron, cobalt, nickel).
10. Optical Properties
- Solids interact with light in ways that can determine their transparency, color, and reflectivity:
- Transparency: Some solids, like glass, allow light to pass through with minimal scattering.
- Reflection: Metals reflect light, giving them a shiny appearance.
- Absorption: Other materials absorb specific wavelengths of light, which contributes to their color.
11. Defects in Solids
- Real solids often contain structural imperfections or “defects” that can impact their properties. Types of defects include:
- Point Defects: Missing or additional atoms.
- Line Defects: Disruptions in the orderly arrangement, like dislocations.
- Surface Defects: Irregularities on the surface or at grain boundaries.
12. Applications in Technology and Materials Science
- The study of solid-state materials underpins a wide range of technologies. Solid-state devices, such as transistors, diodes, and integrated circuits, are critical for electronics. Advanced materials used in construction, aerospace, and medical technology also rely on solid-state properties.
10 Questions and Answers on the General Characteristics of the Solid State, Covering key concepts:
Q: What defines a solid state?
A: In the solid state, particles (atoms, ions, or molecules) are closely packed in a fixed structure, giving solids a definite shape and volume.
Q: Why do solids have a definite shape and volume?
A: Solids have a definite shape and volume because their particles are tightly packed and held by strong intermolecular forces, restricting their movement to vibrations around fixed positions.
Q: How do crystalline and amorphous solids differ?
A: Crystalline solids have an orderly, repeating arrangement of particles and a sharp melting point, while amorphous solids have a random particle arrangement and no definite melting point.
Q: What is meant by “long-range order” in solids?
A: “Long-range order” refers to the regular, repeating arrangement of particles over large distances within crystalline solids.
Q: What does “anisotropy” mean in the context of solid-state materials?
A: Anisotropy means that a crystalline solid’s physical properties, like refractive index and conductivity, vary based on the direction in which they are measured.
Q: Why do crystalline solids have sharp melting points?
A: Crystalline solids have sharp melting points because their particles are arranged in a fixed, regular pattern, requiring a specific temperature to overcome the forces holding them in place.
Q: How do particles in amorphous solids differ from those in crystalline solids?
A: In amorphous solids, particles are arranged randomly without a long-range pattern, leading to isotropic properties and gradual softening over a temperature range instead of a sharp melting point.
Q: What are the main types of crystalline solids based on bonding?
A: Crystalline solids can be ionic (e.g., NaCl), covalent (e.g., diamond), metallic (e.g., copper), or molecular (e.g., ice).
Q: Why are solids generally incompressible?
A: Solids are incompressible because their particles are packed closely together, leaving minimal space between them, making it difficult to compress them further.
Q: What is meant by “isotropy” in amorphous solids?
A: Isotropy means that amorphous solids have identical physical properties in all directions, due to the lack of a structured particle arrangement.