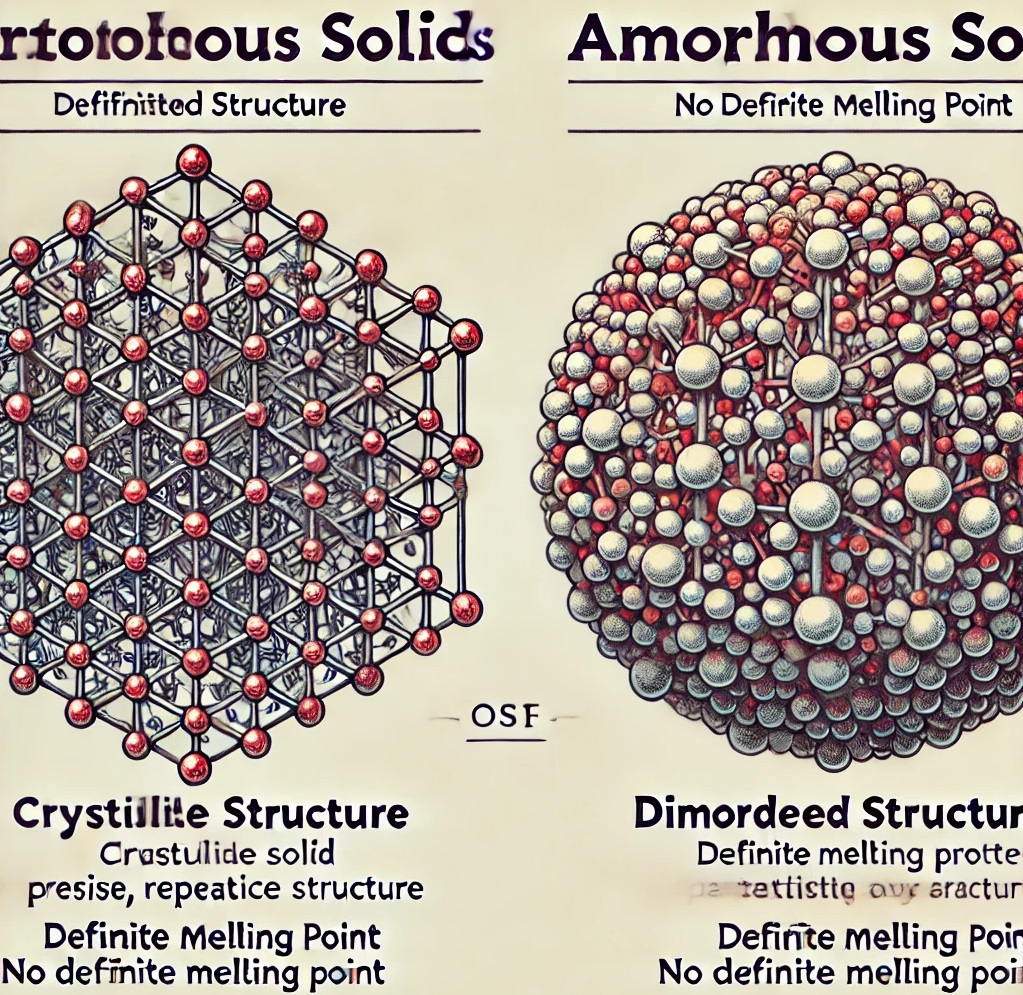
1. Crystalline Solids
Definition: Crystalline solids have particles (atoms, ions, or molecules) arranged in a regular, repeating pattern extending in all three spatial dimensions.
Properties:
- Long-Range Order: Their orderly arrangement extends over large distances, giving them a well-defined geometric shape.
- Sharp Melting Point: Crystalline solids have a definite melting point, melting sharply at a specific temperature.
- Anisotropy: Physical properties such as refractive index, conductivity, and thermal expansion vary based on the direction of measurement, due to their ordered structure.
- Examples: Table salt (sodium chloride), quartz, and diamond are common examples.
Types of Crystalline Solids (based on bonding and structure):
- Ionic Solids: Composed of ions; held by electrostatic forces (e.g., NaCl).
- Covalent (Network) Solids: Atoms connected by covalent bonds in a network (e.g., diamond, silicon carbide).
- Metallic Solids: Positive ions in a “sea” of delocalized electrons (e.g., copper, gold).
- Molecular Solids: Molecules held by intermolecular forces like van der Waals forces, hydrogen bonds (e.g., ice, dry ice).
2. Amorphous Solids
Definition: Amorphous solids lack a regular, long-range arrangement of particles; instead, their structure is more disordered.
Properties:
- Short-Range Order: Particles exhibit order only over short distances, leading to a lack of well-defined shape.
- No Sharp Melting Point: Amorphous solids soften over a range of temperatures rather than melting sharply.
- Isotropy: Physical properties are uniform in all directions since there is no organized structure.
- Examples: Glass, rubber, and plastics are typical amorphous solids.
Nature and Classification:
- Supercooled Liquids: Amorphous solids are sometimes called “supercooled liquids” because they have properties similar to those of liquids.
- Transition to Crystalline: Given enough time or under specific conditions (like heat), some amorphous solids can partially crystallize.
Applications: Crystalline solids are often used in electronics (like silicon in semiconductors) because of their predictable properties, while amorphous solids, like glass, are widely used for their malleable nature and isotropic qualities.
This distinction is crucial in chemistry and materials science as it affects the applications, strength, and stability of materials.
10 Questions Amorphous and crystalline solids
- Q: What is a crystalline solid?
A: A crystalline solid is a type of solid where particles (atoms, ions, or molecules) are arranged in a regular, repeating pattern across the entire structure.Explanation: This orderly arrangement extends over long distances, resulting in defined shapes, clear faces, and predictable properties, as seen in substances like salt, quartz, and diamond.
- Q: What is an amorphous solid?
A: An amorphous solid is a type of solid where particles are arranged randomly, without a long-range repeating pattern.Explanation: Amorphous solids lack a well-defined shape and have irregular particle arrangements, leading to properties similar to liquids in some ways. Examples include glass, rubber, and plastic.
- Q: How do the melting points of crystalline and amorphous solids differ?
A: Crystalline solids have a sharp, definite melting point, while amorphous solids soften over a range of temperatures rather than melting sharply.Explanation: In crystalline solids, the orderly arrangement causes all bonds to break at the same temperature. In contrast, the disordered structure of amorphous solids means bonds have varying strengths, leading to gradual softening.
- Q: What is meant by “long-range order” in crystalline solids?
A: Long-range order means that the particles in a crystalline solid are arranged in a consistent, repeating pattern over large distances.Explanation: This feature gives crystalline solids their characteristic geometry, where a unit cell (smallest repeating unit) is replicated throughout the solid, resulting in a well-defined lattice structure.
- Q: What is the difference in physical properties (anisotropy and isotropy) between crystalline and amorphous solids?
A: Crystalline solids are anisotropic, meaning their physical properties vary with direction, while amorphous solids are isotropic, meaning their properties are the same in all directions.Explanation: In crystalline solids, the ordered structure causes direction-dependent properties, such as varying refractive indices. Amorphous solids, with no long-range order, have uniform properties in all directions.
- Q: What are some examples of crystalline and amorphous solids?
A: Examples of crystalline solids include sodium chloride, diamond, and ice, while examples of amorphous solids include glass, rubber, and plastic.Explanation: These examples show how crystalline and amorphous solids differ in appearance and usage. Crystalline solids have defined shapes, while amorphous solids often have irregular, flexible forms.
- Q: Why are amorphous solids called “supercooled liquids”?
A: Amorphous solids are sometimes called “supercooled liquids” because their disordered particle arrangement is similar to that in liquids, though they appear solid at room temperature.Explanation: This term highlights the similarity in molecular randomness between amorphous solids and liquids, as both lack a fixed, regular pattern.
- Q: How does the rigidity of crystalline solids compare to amorphous solids?
A: Crystalline solids are generally more rigid and stable than amorphous solids due to their ordered structure.Explanation: The strong, repetitive bonds in crystalline solids provide stability, while the irregular structure in amorphous solids often makes them softer or more easily deformed.
- Q: What happens when crystalline and amorphous solids are broken?
A: Crystalline solids break along specific planes, resulting in clean, straight surfaces, whereas amorphous solids break irregularly.Explanation: The orderly arrangement in crystalline solids allows them to cleave along consistent planes. Amorphous solids, lacking such order, fracture unevenly.
- Q: How do crystalline and amorphous solids respond to heating?
A: Crystalline solids absorb heat uniformly until they reach their melting point, where they melt sharply. Amorphous solids absorb heat and gradually soften over a range of temperatures.Explanation: This difference is due to the uniform bond structure in crystalline solids, where all bonds break at once, while the varied bonds in amorphous solids result in a gradual transition.